- Politics, Institutions, and Public Opinion
- Health Care
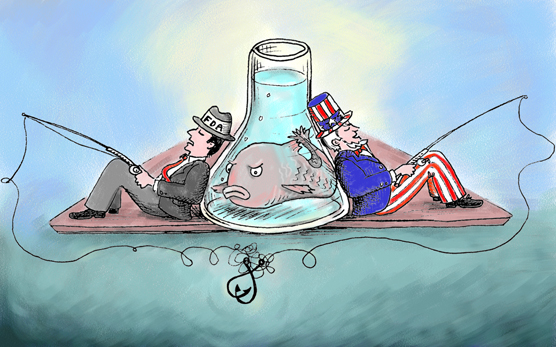
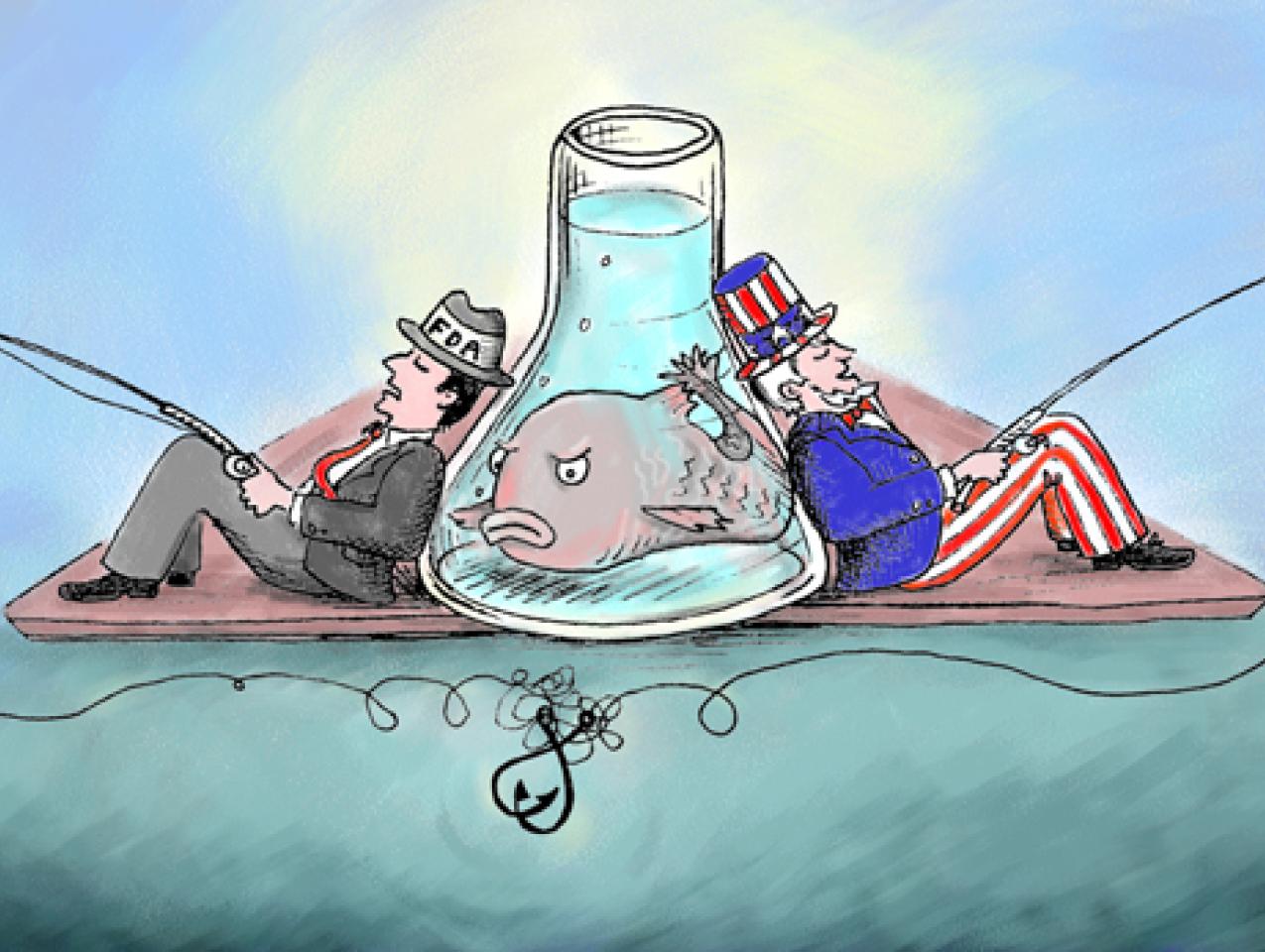
Looking after local interests is an old and venerable tradition in the U.S. Congress; after all, elected officials need to please their constituents. But it can be pushed beyond what is ethical or in the public interest. For instance, members of the House and Senate, hoping to protect special interests back home, are trying to order the Food and Drug Administration (FDA) to stop doing some of the very work it is empowered to do on behalf of American consumers: regulate food safety.
Representatives Don Young (R-AK) and Lynn Woolsey (D-CA) are proposing an amendment that bars regulators from allocating funds to approve a genetically engineered salmon. This comes in spite of an (overly) exhaustive ten-year FDA review that found no hint of any health or environmental problems with the salmon. The bill has passed the House, and Senators are now threatening similar legislation.

Illustration by Barbara Kelley
The congressional rationale? According to the letter: "Given the strong and growing congressional opposition to the approval of [genetically engineered] fish in both chambers spending time on further review of [genetically engineered] fish would be a waste of taxpayer dollars."
But the real waste of taxpayer dollars is congressional interference in a long and scientific regulatory process that is nearing its end, simply to protect the local salmon industry. This is crony capitalism at its worst. Such intolerable meddling in regulation, which is the legitimate province of the Executive Branch of government, is tantamount to legislators from New Jersey organizing an effort to block the approval of a superior new medicine made in California because it would pose an economic threat to a drug made by a New Jersey-based pharmaceutical company.
What makes the congressional action particularly insupportable is that FDA’s process itself was already excessively burdensome. After more than a decade of dithering, in 2008 the FDA's Center for Veterinary Medicine chose to subject every genetically engineered animal intended as food to the same pre-market approval procedures and regulations that drugs used to treat animal diseases undergo. The rationale, according to an FDA policy statement, is that a genetically engineered construct "that is in a [genetically engineered] animal and is intended to affect the animal's structure or function meets the definition of an animal drug." But this explanation conveniently ignores the science, the FDA's own precedents, and the availability of other, more appropriate regulatory options.
Why are Congressmen interfering with the FDA’s scientific regulatory process? Simple. To protect their local salmon industries.
The FDA's existing approach to foods (which is far less lengthy and intensive than that for veterinary drugs) should have been applied to genetically engineered animals intended for consumption. But characteristically, regulators chose the most risk-averse and burdensome procedures.
Genetic engineering of food products is, in fact, old hat. Except for wild game, wild mushrooms, wild berries, and fish and shellfish, virtually all foods in the European and American diet have long been derived from modified organisms—even the organic food at Whole Foods and the local farmers market. Tangelos resulted from a genetic cross between tangerines and grapefruit, for example. Yogurt, beer, tofu, and bread are made with microorganisms that have been painstakingly modified and optimized over the centuries. Grains, in particular, have been intensively engineered over millennia for higher yields, pest- and disease-resistance, and various other desirable characteristics. For example, although modern wheat varieties vary widely in their traits and genetics, all are derived from a common precursor first domesticated in Turkey around 9,000 B.C., and which was subsequently genetically improved by farmers, plant breeders, and biologists. Current varieties include durum wheat for pasta and so-called common wheat for bread.
Animals, too, have been genetically engineered, mostly by laborious and imprecise trial-and-error breeding techniques. The dozens of varieties of cattle raised today are all derived from the now-extinct auroch, which was used both for food and as a beast of burden from ancient times until the seventeenth century. A relatively recent new food animal, the "beefalo," is a cow-bison (buffalo) hybrid. It combines the superior hardiness, foraging ability, ease of calving, and low-fat meat of the bison with the fertility, milking ability, and docility of the cow.
The FDA’s onerous pre-marketing approval requirement applies only if the animal has been modified with state-of-the-art recombinant DNA techniques, also known as "gene splicing." (These techniques involve inserting highly purified and well-characterized segments of DNA into a new host.) Thus, if the fast-maturing Atlantic salmon described above were the result of cruder artificial insemination or irradiation techniques instead of the highly precise genetic engineering methods that were used, it would be exempt from pre-approval evaluation. For instance, the FDA has never regulated food animals, like the beefalo and new strains of fowl, which have been produced with less precise, less predictable methods of genetic modification. The trigger for the FDA’s review is not the risk-related traits of an animal but the use of a certain technology, and the most precise and predictable one at that. That makes no sense.
Lower priced salmon would be a boon for consumers seeking low-fat and affordable options for protein sources.
Even before Congress’ eleventh-hour interference, the FDA’s policy burdened an entire innovative business sector with regulations that inflate research and development costs, inhibit innovation and deprive consumers of less-expensive, healthier products. Not surprisingly, very few companies are willing to swim upstream against such a powerful regulatory current.
The poor fish that has been treading water in regulatory limbo for more than a decade is simply an Atlantic salmon that contains a growth hormone gene from a Chinook salmon. That growth hormone gene is turned on all year instead of only during the warmer months as it is in its unmodified cohorts. As a result, the salmon reaches a marketable adult weight in about half the time it otherwise would.
The genetic change confers no detectable difference in its appearance, ultimate size, taste, or nutritional value; the fish simply grows faster, a tremendous economic advantage to those farming the fish in a closed water system. It’s also an advantage to consumers, who will be able to take advantage of greater supply and lower prices. Lower priced salmon would be a boon for consumers seeking low-fat and affordable options for protein sources, especially in the face of food price inflation and the obesity epidemic.
After an exhaustive analysis, the FDA concluded that the modified salmon has no detectable differences from its unmodified relatives and that it "is as safe as food from conventional Atlantic salmon." In addition, since the fish will be farmed inland and all of its females will be sterile, there is virtually no possibility of negative environmental effects such as "genetic contamination" of the wild Atlantic salmon’s gene pool. (Even in the worst-case scenario, these modified fish would compete poorly in the wild.)
The FDA concluded that the genetically modified salmon is perfectly safe.
But that isn’t good enough for Alaska Senator Mark Begich, a Democrat whose home-state salmon industry could be a beneficiary of a decision to pull the plug on the more efficient, faster growing salmon. He alleged that the "FDA hasn't considered all of the potential negative impacts of genetically altered fish and the strong opposition in Congress to approving something that could decimate wild salmon populations." Rubbish. Some say fish is "brain food"—that is, that eating it makes you smarter. Begich should consider an all-fish diet.
Officials at AquaBounty, the company that created the fast-growing salmon and that favored the FDA’s more burdensome review pathway originally, have become disillusioned. The CEO, Ronald Stotish, lamented:
We hoped that the rigorous science-based review would provide confidence and assurance to the general public that their interests were being protected...In retrospect, however we were naive to assume that the well-organized and well-funded opponents of technology would accept a science-based process. The environmental and organic lobbies ignored the data and the FDA conclusions, and proceeded with their attacks. In my personal view, the ‘transparency’ exercise in political correctness simply provided opponents an opportunity to crucify the company, the FDA, and the science based process.
Others who might be tempted to invest in or create new technologies and products will get the message: even with a superior product, you can be blindsided by cynical political forces as you near the finish line.
This is a sad commentary on innovation in America. The British historian Paul Johnson has written, "Left to themselves, the creative forces in society will always deliver, but keeping them reasonably free to do so is a perpetual grinding battle. It is one that must never be lost." Once again, certain members of Congress are fighting on the wrong side of that battle.