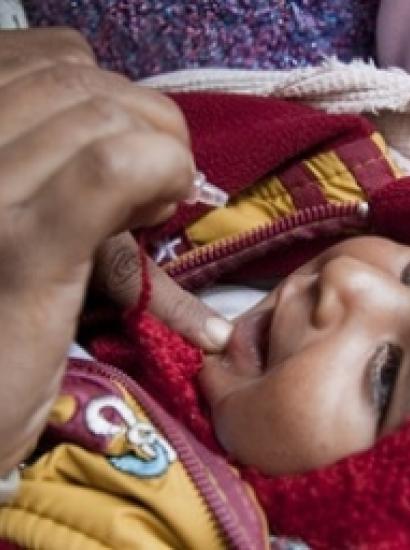
- Health Care
“If we can save only one child’s life…” is a phrase frequently used to justify one initiative or another. It has been invoked in recent years to promote causes ranging from the installation of seatbelts in school buses to anti-alcohol campaigns directed at pre-teens. But when it comes to saving lives through certain infant vaccinations, public health officials seem not to grasp the concept.
Consider meningococcal disease, a rare but devastating bacteria-caused illness that primarily affects infants and children. Its elimination has been on the U.S. Centers for Disease Control’s list of priorities since 1999, but in early 2010, around the same time that a vaccine to prevent meningococcal disease in infants was submitted to the FDA for approval, the CDC began to show signs of retreating from its earlier resolve. Its motives are unclear.
Recommendations for children’s immunization schedules come from the CDC’s Advisory Committee on Immunization Practices (ACIP). Last year, the Meningococcal Working Group, made up of experts who advise the ACIP, considered a recommendation not to perform routine infant vaccination for meningococcal disease, ostensibly because the burden of the illness in the United States is “minimal” and does not justify the expense of adding the vaccine to the infant immunization schedule.

Infant receiving a vaccine (Photo credit: Gates Foundation)
How does one define a minimal burden? The ACIP report doesn’t specify, and many infectious disease experts would beg to differ with that characterization. Meningococcal disease is both unpredictable and deadly, killing 10 to 15 percent of those who contract it. Among those who survive, as many as one in five will suffer permanent disability from amputations, seizures, paralysis, hearing loss, and learning disabilities—hardly a “minimal burden” for the individuals and the families affected—or for a health care system that shares the costs of lifelong disability. The onset of the disease is rapid and insidious, and it can be fatal within 24 to 48 hours of the first non-specific symptoms.
The narrow window of time to diagnose and successfully treat a patient makes the treatment—instead of the prevention—of meningococcal disease a dubious strategy. That’s why vaccination is so important. Given the success of current U.S. vaccine programs, which have almost eliminated many other infectious diseases, the prevention of meningococcal disease qualifies as an unmet public health need.
The goal of the U.S. vaccination program should be to eliminate meningococcal disease. The preferred strategy to eliminate the disease would include infant immunization, even though—for reasons that are unknown—the incidence of the disease has declined in recent years. In 2007, the CDC estimated that there were 1,000 cases and 130 deaths nationally, compared with 2,800 cases and 300 fatalities a decade earlier. History has shown, however, that spikes in the incidence of meningococcal disease can occur over a short time period and without warning. Moreover, the prevailing strains of meningococcus could evolve to become more virulent. What the ACIP considers a minimal burden could increase rapidly, with dire consequences.
Meningococcal disease is both unpredictable and deadly.
The absence of satisfactory treatment options combined with the high risks for infants makes a compelling argument for aggressive protection against the disease in the United States through infant vaccination, which would complement the existing adolescent immunization strategy. The latter ensures retention of immunity.
So who should be vaccinated? The Public Health Agency of Canada calls for use of a meningococcal vaccine as early as two months of age. However, in the United States, the current immunization schedule for this disease begins at two years of age for high-risk children (of whom there are very few) and is recommended for all at age 11. A vaccine for infants as young as two months of age was approved by the FDA in April, but the CDC appears to be wavering on whether to recommend its use for the routine vaccination of infants in spite of its own recent data indicating they are at high risk: The incidence of meningococcal disease is three to seven times higher in infants under one year old than in any other age group.
Many industrialized nations, including Australia, Canada, and 13 European nations, consider the threat of meningococcal disease to be sufficiently high to recommend routine infant vaccinations. The results have been impressive: According to the World Health Organization’s Initiative for Vaccine Research, the 12-year-old infant vaccination program in the U.K. has “had a tremendous impact on the incidence of the disease, resulting in a more than 90% decrease in the number of deaths and clinical cases.” (It is significant that that lower incidence of infections was found only in the single meningococcal strain that was contained in the vaccine. That offers assurance that the reduction in illness resulted from vaccination, not from a lower ambient incidence of meningococcal infections.)
Also according to WHO, the inclusion of the meningococcal vaccine in infant vaccination programs in the Netherlands and Canada has shown efficacy of 87 to 98 percent in various studies. And in millions of patients, meningococcal vaccines have demonstrated excellent safety profiles, like those of other routinely administered vaccines.
Vaccination against meningococcal infection is a public health imperative, but instead of placing a high priority on the elimination of this disease by promoting vaccination at an early age and ensuring that patients and parents have access to state-of-the-art vaccines, the CDC has been dithering. The possible reasons are worth exploring because they could apply to a wide range of decisions that the government will have to make as it plays a larger role in the funding of healthcare.
We need a strategy of prevention—not treatment—for this disease.
The fundamental question is how public health officials make decisions that are essentially scientific and economic. (Unlike the FDA, which considers only the safety and efficacy of vaccines, the CDC can consider cost as well.) Well, you might think the process would involve the convening of panels of internal and external experts in pediatric virology, pediatrics, epidemiology, and medical economics to deliberate the critical questions. You would be wrong.
The CDC recently held a series of carefully orchestrated public meetings across the nation—from New Hampshire to Washington State—supposedly to obtain input from non-experts. CDC spokesman Glenn Nowak explained that the organization views the issue from a “population health” perspective. In other words, the CDC is trying to ascertain whether to recommend the vaccination, weighing the benefit not only against the cost but also, as Nowak put it, against the already strained public health infrastructure. This contrasts, he said, with the role of the FDA, which determines only whether the vaccine is safe and effective. He added that the CDC and its ACIP have few options; they can make an addition to the schedule of immunizations, endorsing it for everyone who does not have a contraindication, or recommending it for a subset of the population, such as children or the elderly. Or they can leave the decision about who gets the vaccine to individual medical practitioners, which is equivalent to making no recommendation at all.
In order to answer the myriad questions involved in these decisions, said the CDC’s Nowak, “we went out and took interested citizens and got their input” on a range of topics, from vaccines in general to the meningococcal disease vaccine in particular. From extra-governmental accounts of these public meetings (no minutes were made available), it is clear that they were conducted like a “push poll,” in which the questions were intended to impart, rather than obtain, information, and to persuade citizens of certain views.
For example, one question posed to the public attendees was, “Thinking about vaccine recommendations, how many infants and children would need to get a severe illness from the disease in a typical year in the U.S. to make it a good idea that all children be vaccinated?” Another was, “If you had to pay for a vaccine that protected your child from a rare but serious disease, what would you be willing to pay out of pocket?”
According to Kendall Antekeier, a staffer from the Heartland Institute who attended the Chicago meeting, the CDC’s agenda became even more evident from the presentation of Dr. Amanda Cohn, its lead expert for domestic meningococcal disease. Antekeier related that the “main objective” of Cohn’s presentation was to convince participants that: (1) “[t]he vaccine would not save ‘that many’ children”; (2) “it may not be ‘cost-effective,’ a term used often by all CDC representatives”; and (3) “the vaccine may clutter an already full vaccination schedule.”
The CDC should not be seeking public input on complex scientific issues.
Yet, according to Antekeier, in spite of the slant of the presentations and questions, “the majority of participants voted that cost should not matter ‘at all’ when recommending vaccine use, and 86% of participants voted for the CDC to recommend the vaccine.” (The CDC refused to divulge the results of these polls, perhaps because the outcome was not what officials were hoping for—namely, to make a case against adding the vaccine to the schedule.)
There are several problems with the CDC’s approach to the critical decision about whether to add this vaccine to the recommended schedule. First, public input on excruciatingly complex scientific and economic issues is of dubious value. Putting it another way, public endorsement is neither necessary nor sufficient on issues of scientific policy. Government scientists and administrators should make decisions based on the evidence and then explain and defend those decisions to the public, repeatedly if necessary.
Second, this preoccupation with sampling public opinion wastes time and taxpayer money.
Third, and perhaps most troubling, having decided to hold the meetings, the bureaucrats apparently tried to use them to sway the public participants toward a specific preordained outcome.
We can only conclude that this vox populi exercise was an elaborate charade conducted by the CDC in order to justify its withholding of the meningococcal vaccine from the schedule for children under two years old. The motive? Possibly the fear that an additional vaccine that “would not save that many children” would feed the skepticism in some quarters about “an already full vaccination schedule.” Or perhaps it derives from a misguided concern about the vaccine not being cost-effective.
These motivations hardly justify a decision not to add the meningococcal vaccine to the immunization schedule. That decision would result in children suffering otherwise preventable death or disability.
There is a financial as well as a medical aspect to such decisions; given the high development costs of vaccines as well as the imposing regulatory barriers, without a CDC mandate to ensure a large and sustained market for vaccines, industry will lack the incentive to invest in vaccines that won't protect a large swath of the population. This does not bode well for research and development.
Government bureaucrats usually hold others to strict standards, rules, and prescribed procedures. This administration, in particular, has repeatedly promised to set a high bar for honesty and transparency. How ironic, then, that this “public” process has been so murky and problematic.